why is the boiling point of hydrogen sulfide low What did scientists expect as the water’s boiling point without
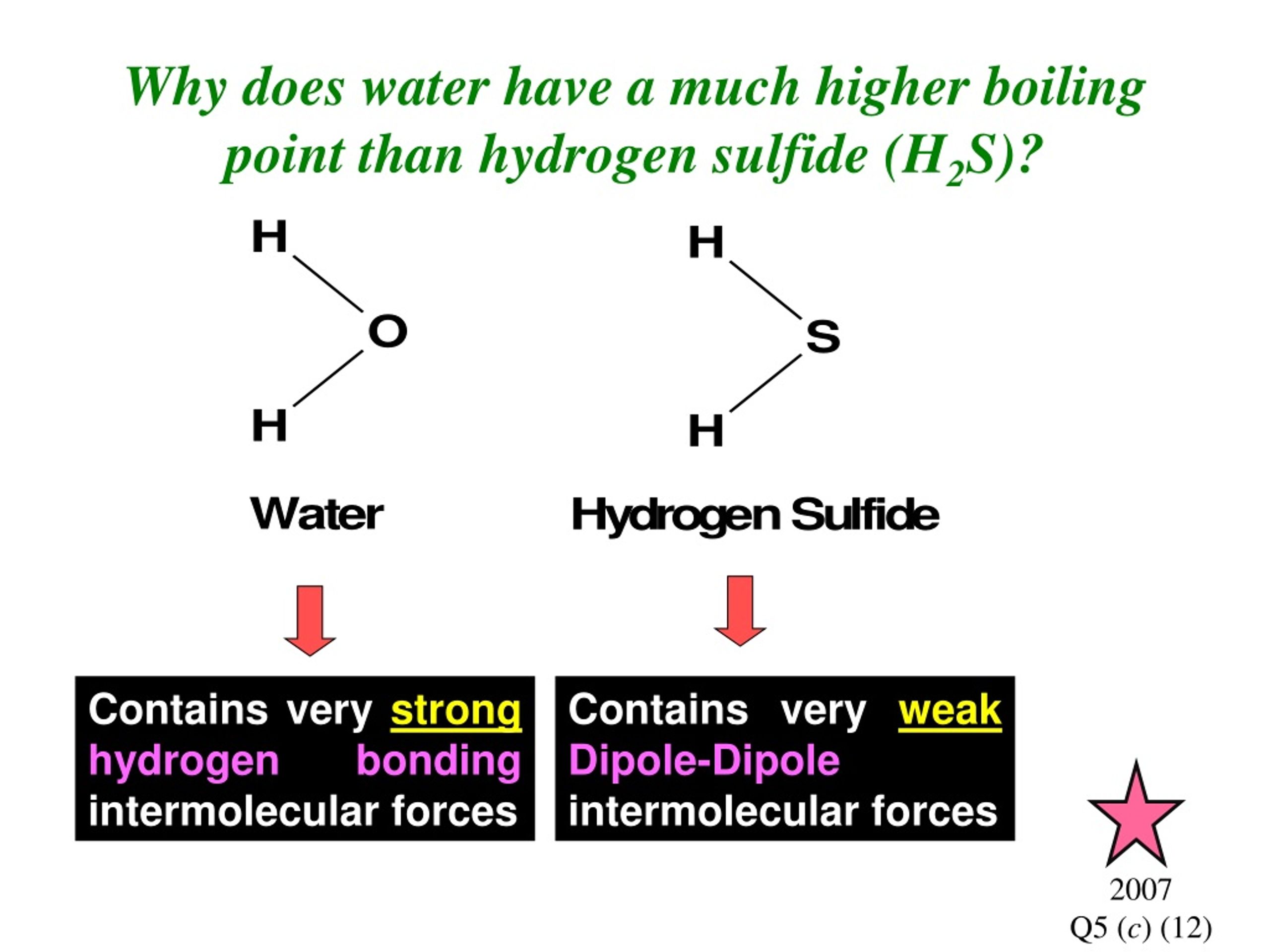
The boiling point of water is greater than hydrogen sulphide which is a topic that many students in science classes often come across. Boiling point is the temperature at which a substance changes its state from liquid to gas, and it depends on several factors such as molecular weight, intermolecular forces, and the structure of the molecule. However, when comparing two substances, the difference in boiling points can be attributed to the intermolecular forces since they affect how tightly the molecules are held together.
Water vs Hydrogen Sulphide
 Water molecules are held together by hydrogen bonds, which are the strongest of all intermolecular forces. Hydrogen sulphide molecules are held together by covalent bonds, which are weaker than hydrogen bonds. Therefore, the strength of the intermolecular forces in water is greater than hydrogen sulphide, which results in a higher boiling point for water. Hydrogen sulphide has a boiling point of -60.4°C, while water has a boiling point of 100°C at standard atmospheric pressure.
Water molecules are held together by hydrogen bonds, which are the strongest of all intermolecular forces. Hydrogen sulphide molecules are held together by covalent bonds, which are weaker than hydrogen bonds. Therefore, the strength of the intermolecular forces in water is greater than hydrogen sulphide, which results in a higher boiling point for water. Hydrogen sulphide has a boiling point of -60.4°C, while water has a boiling point of 100°C at standard atmospheric pressure.
Hydrogen Halides
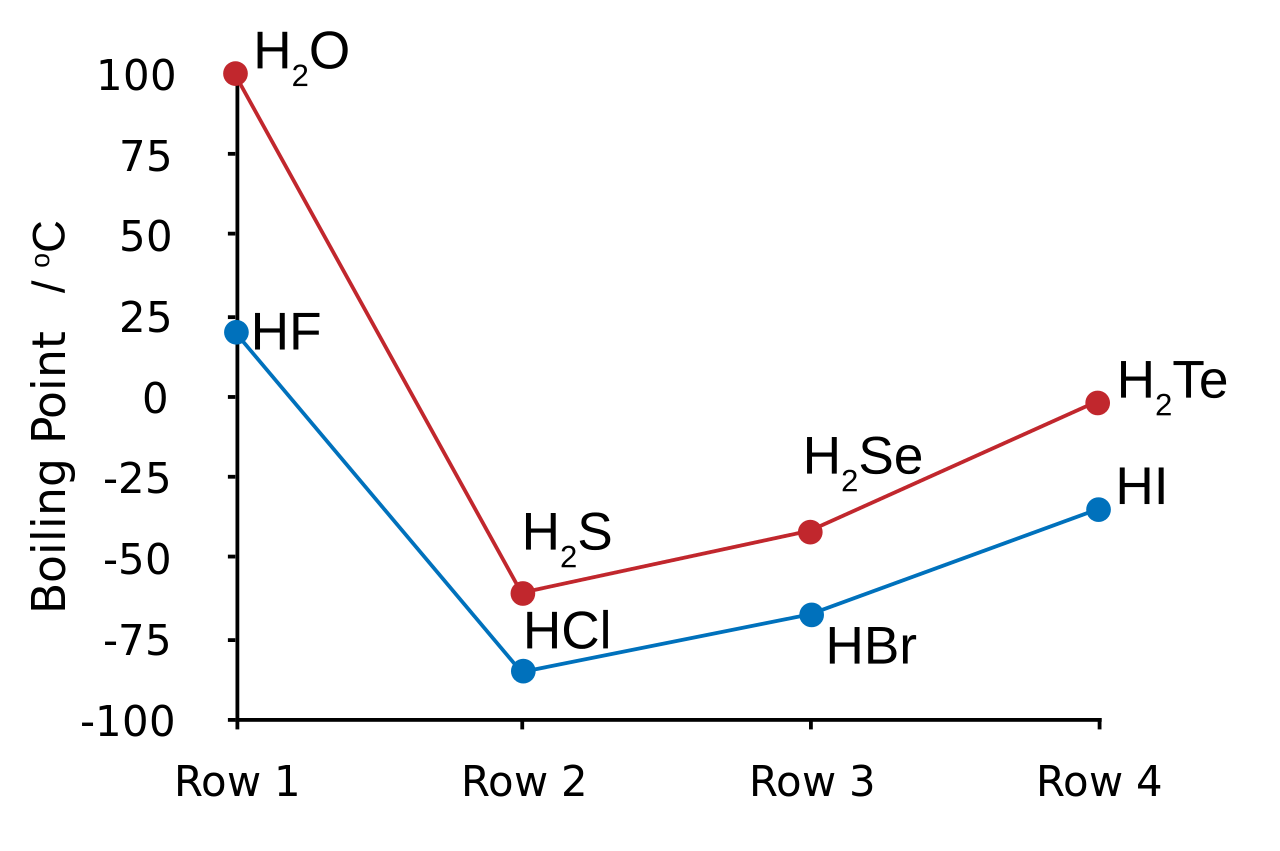 Hydrogen halides are a group of gaseous compounds that are covalently bonded and have high boiling points. They include hydrogen fluoride, hydrogen chloride, hydrogen bromide, and hydrogen iodide. The boiling points of these compounds increase as the molecular weight increases. However, the boiling point of each compound also depends on the strength of the intermolecular forces between the molecules.
Hydrogen halides are a group of gaseous compounds that are covalently bonded and have high boiling points. They include hydrogen fluoride, hydrogen chloride, hydrogen bromide, and hydrogen iodide. The boiling points of these compounds increase as the molecular weight increases. However, the boiling point of each compound also depends on the strength of the intermolecular forces between the molecules.
Hydrogen fluoride has the highest boiling point among the hydrogen halides because it has the strongest intermolecular forces. This is because hydrogen fluoride molecules have a very high polarity due to the small size of fluorine and the electronegativity difference between hydrogen and fluorine. The boiling point of hydrogen fluoride is -83.6°C.
Hydrogen chloride has a lower boiling point than hydrogen fluoride because the size of chlorine is larger than fluorine and the electronegativity difference between hydrogen and chlorine is smaller than hydrogen and fluorine. The boiling point of hydrogen chloride is -85.05°C.
Hydrogen bromide and hydrogen iodide have even lower boiling points than hydrogen chloride because the size of the halogens gets larger and their electronegativity difference with hydrogen gets smaller. The boiling points of hydrogen bromide and hydrogen iodide are -66.8°C and -35.36°C, respectively.
In conclusion, boiling points of compounds depend on their intermolecular forces, which are affected by their molecular weight, structure, and polarity. Water has a higher boiling point than hydrogen sulphide because the intermolecular forces in water are stronger than in hydrogen sulphide due to hydrogen bonding. Hydrogen halides have higher boiling points as the molecular weight increases and the polarity of the molecule increases due to decreasing size of the halogen in the molecule.
If you are searching about The liquefied hydrogen halides have the normal boiling points given you’ve came to the right web. We have 5 Pictures about The liquefied hydrogen halides have the normal boiling points given like Science blog: Vocabulary: Solid, Liquid , and gases, The liquefied hydrogen halides have the normal boiling points given and also Science blog: Vocabulary: Solid, Liquid , and gases. Read more:
The Liquefied Hydrogen Halides Have The Normal Boiling Points Given
 socratic.orgboiling points hydrogen hf halides highest water point hcl hi hbr than why lowest bonds which graph bonding hydrides fluoride
socratic.orgboiling points hydrogen hf halides highest water point hcl hi hbr than why lowest bonds which graph bonding hydrides fluoride
Q. Why Boiling Point Of Water Is Greater Than Hydrogen Sulphide?( Class
 www.youtube.comhydrogen boiling point
www.youtube.comhydrogen boiling point
What Did Scientists Expect As The Water’s Boiling Point Without
www.quora.comboiling hydrides point points hydrogen water why than much group graph hydride does fluoride groups higher bonding ammonia estimate high
PPT - 7 – Shapes Of Molecules & Intermolecular Forces PowerPoint
 www.slideserve.comboiling intermolecular h2s hydrogen molecules powerpoint
www.slideserve.comboiling intermolecular h2s hydrogen molecules powerpoint
Science Blog: Vocabulary: Solid, Liquid , And Gases
 michelleconcepcion.blogspot.comboiling point chemistry water hydrogen liquid science temperature gas bonds bond molecules definition heated evaporation state changes heat into solid
michelleconcepcion.blogspot.comboiling point chemistry water hydrogen liquid science temperature gas bonds bond molecules definition heated evaporation state changes heat into solid
Q. why boiling point of water is greater than hydrogen sulphide?( class. Boiling points hydrogen hf halides highest water point hcl hi hbr than why lowest bonds which graph bonding hydrides fluoride. Science blog: vocabulary: solid, liquid , and gases